Organic & Biomolecular Chemistry
Hardesh K. Maurya , Sanjay K Gautam , Ramendra Pratap , Vishnu K. Tandon , Abhinav Kumar , Vikas Bajpai , Brijesh Kumar and Vishnu Ji Ram
Org. Biomol. Chem., 2012, 10, 4977
DOI: 10.1039/C2OB25173FThe
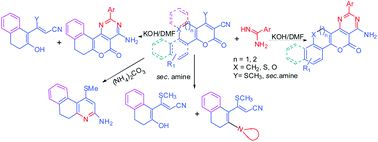
synthesis of three new classes of heteroarenes, built through the
sequential fusion of naphthalene, benzo/naphtho[b]oxepine and
thiochromene rings with pyran and pyrimidine ring system to give ‘U and
Z’ shapes of structural frame work is reported. The methodology is based
on the synthesis of pyran fused intermediates,
1-methylthio-3-oxo-5,6-dihydro-3H-benzo[f]chromene-2-carbonitrile (3),
4-methylthio-2-oxo-5,6-dihydro-2H-benzo/naphtho[b]pyrano[2,3-d]oxepine-3-carbonitriles
(10, 20) and
4-methylthio-2-oxo-2,5-dihydrothiochromeno[4,3-b]pyran-3-carbonitriles
(15) from the reaction of 2-tetralone, benzo/ naphtho[b]oxepin-5-ones
and thiochromen-4-ones with methyl 2-cyano-3,3-dimethylthioacrylate
respectively. Further condensation of intermediates 3, 10, 20 and 15
with amidines led to the formation of tetracyclic ‘U’ shaped
4-amino-2-aryl-7,8-dihydro-5-oxo-5H-naphtho[2,1-b]pyrimido[4,5-d]pyrans
(8) and ‘Z’ shaped
4-amino-2-aryl-5-oxo-12,13-dihydro-5H-benzo/naphtho[b]oxepino[5,4-b]pyrimido[4,5-d]pyrans
(12, 22) and
4-amino-2-aryl-5-oxo-5,12-dihydrothiochromeno[4,3-b]pyrimido[4,5-d]pyrans
(17). Compound 12f forms chain of dimers through N-H∙∙∙O interactions
as indicated by the X-ray structure analysis and the quantum chemical
calculations performed at the MP2 level indicates that this interaction
energy is 10 kJ mol.-1