Hardesh K. Maurya, Vishnu K. Tandon, Brijesh Kumar, Abhinav Kumar, Volker Hüch and Vishnu Ji Ram
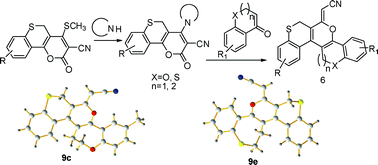
An efficient and convenient route for the construction of helical ‘S’ shaped dioxathia- and oxadithiahelicenes with oxygen and sulfur atoms located in the middle of the outer helix has been developed through base induced inter-, and intramolecular C-C bond formation from the reaction of 4-sec.amino-2-oxo-2,5-dihydrothiochromeno[4,3-b]pyran-3-carbonitriles with 3,4-dihydro-2H-benzo[b]oxepin-5(2H)-ones, 3,4-dihydrobenzo[b]thiepin-5(2H)-one and thiochroman-4-ones separately. Quantum chemical calculations have also been carried out to explore the geometries and electronic structures of newly synthesized compounds to envisage the pathway for interconversion of both atropisomers. The determination of helicity parameters and configurational stability demonstrate that the energy barrier is strongly dependent on the nature of hetero atoms present.

No comments:
Post a Comment