- Tetrahedron Letters, In Press, Accepted Manuscript, Available online 28 September 2014
- Vishnu K. Tandon,
- Manoj K. Verma,
- Hardesh K. Maurya,
- Sandeep Kumar
-
- Received 23 July 2014, Revised 20 September 2014, Accepted 22 September 2014, Available online 28 September 2014
Abstract
An efficient, novel and concise one pot regio- and chemoselective synthesis of benzo[a]phenazines (4) and naphtho[2,3-d]imidazoles (8) has been accomplished in excellent yields by nucleophilic substitution reaction of 2,3-dichloro-1,4-naphthoquinone (1) with o-phenylenediamine (2) and benzamidines (7) respectively “in H2O” using base and micelles (SDS) as catalyst. Analogues reaction of 2,3-dichloro-1,4-naphthoquinone (1) with 2-aminobenzenethiol (9) under identical conditions led to formation of a mixture of benzo[b]phenothiazine (10), benzo[a]phenothiazine (11) and benzo[a]-1,4-benzothiazino-3,2-phenothiazine (12) in 17, 23 and 57% yields respectively.
-
This blog is summarized my research work and publications in Heterocyclic and Medicinal Organic Chemistry
Tuesday, September 30, 2014
Tuesday, May 13, 2014
Boron tribromide mediated C-C bond formation in cyclic ketones: a transition metal free approach
Imran Ahmad, Vinay Pathak, Prema G Vasudev, Hardesh K. Maurya and Atul Gupta
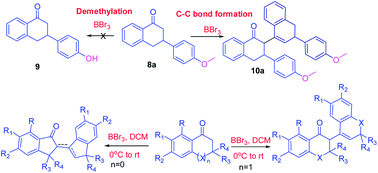
RSC Adv. 2014, 4, 24619
Accepted 12 May 2014
First published online 13 May 2014
Abstract
Borontribromide (BBr3) is a well known demethylating agent. Current investigation was focused on a new application of borontribromide as C-C bond forming agent in cyclic ketones. In this study, borontribromide mediated C-C bond formation reactions of tetralones, chromenone, thiochromenone and indanones were explored. Methoxy group containing ketones showed selective C-C bond formation reaction instead of demethylation of methoxy group. MM2 steric energy calculations for final products showed that reaction favored the formation of exo or endo cyclic double bond containing products depending upon their low MM2 steric energy in a specific frame structure as observed in x-ray crystallography. A comprehensive crystallographic and pi-stacking analysis of product 10a exhibited the formation of 10a as enantiomeric mixture and its centre of inversion was stabilized by a set of three unique pi-pi interactions.

Wednesday, May 7, 2014
A convenient synthesis of pyrimidinone and pyrimidine containing bisheteroarenes and analogs
Hardesh K. Maurya and Atul Gupta
RSC Adv., 2014, 4, 22106-22114
DOI: 10.1039/C4RA01689K
Received 26 Feb 2014,
Accepted 02 May 2014
First published online 07 May 2014
Abstract
The synthesis of pyrimidinone containing bisheteroarenes (3) and related analogs (9 and 10) by the reaction of active methylenes or substituted methyl acrylate with nitrogen containing precursors viz amidines, or thiourea in water as well as other organic solvents was studied. Synthesized compounds had further been explored for the synthesis of diversified pyrimidines 4, 6-8, 11-12 and 14 through sequential approach.
Monday, May 5, 2014
Regioselective synthesis of polycyclic aza-oxa and aza-oxa-thia heteroarenes as Colo-205 and HepG2 carcinoma cells growth inhibitors
European Journal of Medicinal Chemistry,Volume 81, 23 June 2014, Pages 367–377 Available online 5 May 2014
Hardesh K. Maurya, Sanjay K. Gautam, Ramendra Pratap, Vishnu K. Tandon, Abhinav Kumar, Brijesh Kumar, Shruti Saxena, Deepti Tripathi, Meenakshi Rajwanshi, Mukul Das, Vishnu Ji Ram

Hardesh K. Maurya, Sanjay K. Gautam, Ramendra Pratap, Vishnu K. Tandon, Abhinav Kumar, Brijesh Kumar, Shruti Saxena, Deepti Tripathi, Meenakshi Rajwanshi, Mukul Das, Vishnu Ji Ram
DOI: http://dx.doi.org/10.1016/j.ejmech.2014.05.013
Abstract
An efficient regioselective synthesis of polycyclic diheteroaryl[b,d]pyrans and diheteroaryl[c,e][1,2]diazepines has been reported through ring transformation reactions of 2-oxo-2,5-dihydrothiochromeno[4,3-b]pyrans (3,4), 2-oxo-5,6-dihydro-2H-benzo[b]pyrano[2,3-d]oxepine/thiepine (8, 9) and 6-oxo-3,6-dihydro-2H-naphtho[1,2-b]pyrano[2,3-d]oxepine (15) by hydrazine, at ambient and reflux temperature. Nine compounds viz 5a,b; 10a,c,d; 12b; 13b; 16 and 1-methylthio-5,6-dihydrobenzo[f]quinoline (0.1-100 μM) were screened for their cytotoxicity in normal (IEC-6), carcinoma (Colo-205) and Hep G2 cell lines. None of the compounds showed cytotoxicity in normal IEC-6 cells while 10a,d and 16 resulted in killing of Colo-205 cells with IC50 ranging between 20-60μM while 10c and 13b caused killing of HepG2 cells with IC50 values ranging between 60-80 μM concentration. Further, 10a,d and 16 caused apoptosis through a cascade of mitochondrial pathway in Colo-205 cells indicating anticancerous potential against intestinal cancer. Interestingly, compounds 10c and 13b exhibited apoptosis through mitochondrial pathway in HepG2 cells suggesting anticancer activity against hepatic cancer.
Synthesis and biological evaluation of substituted 4,6-diarylpyrimidines and 3,5-diphenyl-4,5-dihydro-1H-pyrazoles as anti-tubercular agents
Bioorganic & Medicinal Chemistry Letters, Volume 24, Issue 13, 1 July 2014, Pages 2892–2896, Available online 4 May 2014
Vinay Pathak, Hardesh K. Maurya, Sandeep Sharma, Kishore K Srivastava, Atul Gupta

Vinay Pathak, Hardesh K. Maurya, Sandeep Sharma, Kishore K Srivastava, Atul Gupta
DOI: http://dx.doi.org/10.1016/j.bmcl.2014.04.094
Abstract
Various substituted 4,6-diarylpyrimidin-2-amine (4), 4,6-diaryl-2-(heteroaryl)pyrimidine (6) and 1-(3,5-diaryl-4,5-dihydro-1H-pyrazol-1-yl)ethanone (7) derivatives were synthesized in good yields using simple methodology. The synthesized compounds (4-7) were evaluated for their in vitro anti-tubercular activity against Mycobacterium tuberculosis H37Rv strain. Compounds 4a, 6b, 7b, and 7c exhibited significant anti-tubercular activity at MIC values 25, 25, 12.5 and 12.5 μM concentration. In vitro cytotoxicity data using non cancerous hepatic monocytes (THP-1) cells indicated that most active compounds 7b and 7c were safe as their MIC values were much lower than their cytotoxic values

Thursday, February 20, 2014
Synthesis and evaluation of 2-heteroaryl and 2,3-diheteroaryl-1,4-naphthoquinones that potently induce apoptosis in cancer cells
Vishnu K. Tandon, Hardesh K. Maurya, Sandeep Kumar, Aijaz Rashid and Dulal Panda
This article describes the preparation of 2-heteroaryl and 2,3-diheteroaryl-1,4-naphthoquinones by an environmentally benign short synthetic route with the goal of finding 1,4-naphthoquinone derivatives that induce apoptosis in cancer cells. We have identified three most active naphthoquinones 10, 12 and 15 that potently induce apoptosis in human cervical carcinoma (HeLa) cells. One of these three compounds perturbed both microtubule and actin filaments.
Monday, February 3, 2014
A carbanion induced synthesis of highly congested pyrazole and imidazole containing heterocycles
Tetrahedron Letters, Volume 55, Issue 10, 5 March 2014, Pages 1715-1719
Hardesh K Maurya, Atul Gupta
Medicinal Chemistry Department, Central Institute of Medicinal and Aromatic Plants, P.O. CIMAP, Kukrail Road, Lucknow-226015, India
http://dx.doi.org/10.1016/j.tetlet.2014.01.095
Hardesh K Maurya, Atul Gupta
Medicinal Chemistry Department, Central Institute of Medicinal and Aromatic Plants, P.O. CIMAP, Kukrail Road, Lucknow-226015, India
http://dx.doi.org/10.1016/j.tetlet.2014.01.095
Abstract
An efficient approach to the synthesis of highly congested di, penta and hexacyclic pyrazoles as well as imidazole fragment containing novel heterocyclic molecule has been developed through a carbanion induced transformation of suitably functionalized 2H-pyran-2-ones, benzo[h]chromene and thiochromeno[4,3-b]pyrans. Due to the presence of fluorescence, we report their prime application metal sensor as off/on switching in ferric ions.
Subscribe to:
Comments (Atom)

